Community
osapeers.org
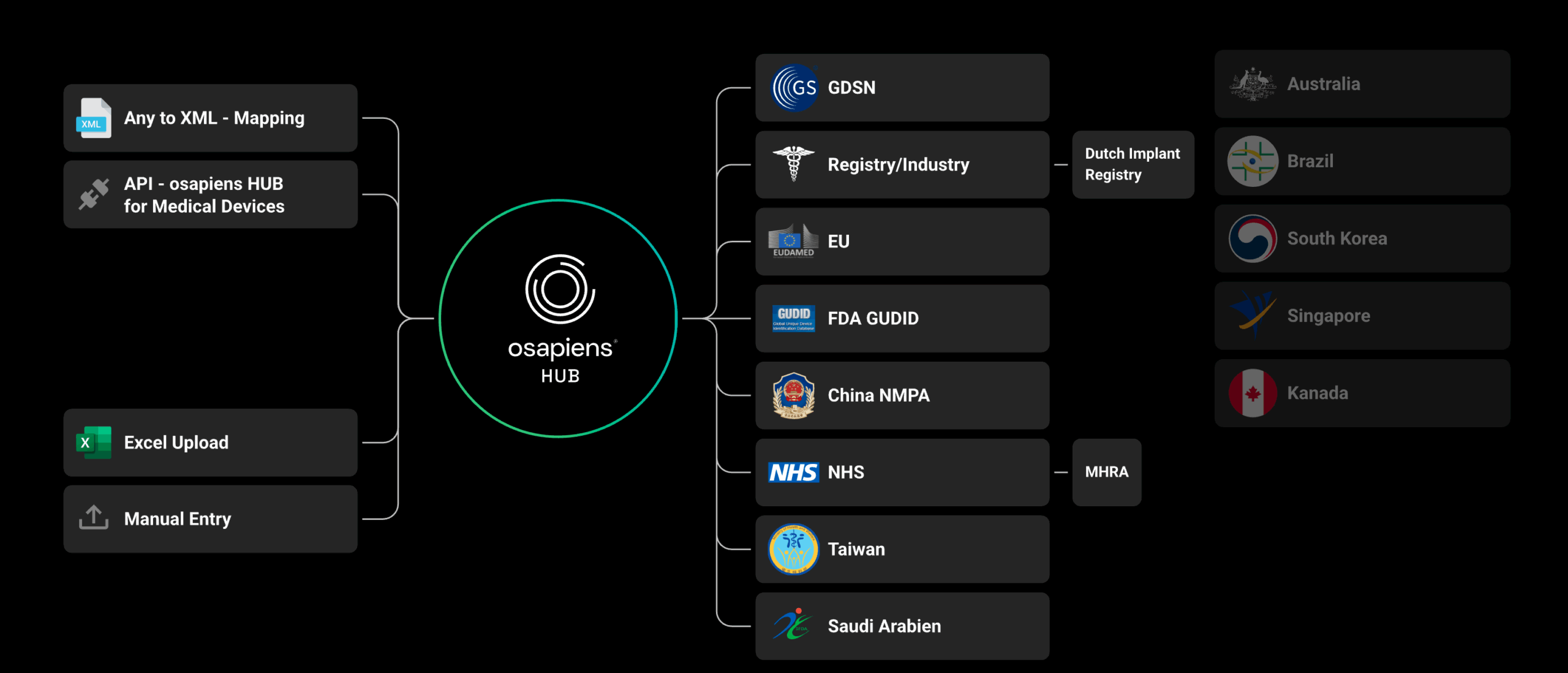
For medical device manufacturers, complete and accurate product data are essential to meet both regulatory and commercial trading partner requirements. Regulations such as EUDAMED (European Commission) and GUDID (US FDA) require the submission of comprehensive product information to their respective databases before products can be marketed. This data forms the foundation of a safe and secure healthcare supply chain, enabling more reliable post-market surveillance and significantly improving patient safety.
The osapiens platform supports regulatory requirements including EUDAMED, GUDID, SFDA (Saudi-DI), TFDA (Taiwan), and the Netherlands LIR (via GDSN), as well as upcoming requirements from TGA Australia, Swissmedic, Emirates Health Services (EHS), and Dubai Health Authority (DHA).
By automating data exchange, osapiens reduces manual effort across the entire product lifecycle, streamlining compliance and improving operational efficiency.