Community
osapeers.org
This article is available in
About this article

Hardik Agrawal
Authorswissdamed (Swiss Database on Medical Devices) is Swissmedic’s national database for medical devices in Switzerland. It is used to register economic operators and medical devices that are placed on the Swiss market, including in vitro diagnostic medical devices (IVDs). The goal is to make key information easier to find and to support transparency, traceability, and regulatory oversight.
swissdamed also includes a public website with search functions. This lets users look up medical devices available in Switzerland and see which companies are responsible for them, such as manufacturers, authorized representatives, and importers.
swissdamed was created after a major regulatory change in May 2021. When the Mutual Recognition Agreement (MRA) between Switzerland and the EU ended, Switzerland could no longer rely on automatic recognition of EU medical device approvals. Switzerland therefore had to adapt its approach to keep safety standards aligned with the EU while using its own national system.
In this context, swissdamed was developed as Switzerland’s counterpart to EUDAMED, the European database. It supports Switzerland’s regulatory oversight while staying compatible with EU systems where needed.
swissdamed was designed to bring structure and clarity to the Swiss medical device landscape.
swissdamed follows a modular structure. This means that different types of data are handled in dedicated modules rather than one large system. The approach mirrors the structure of EUDAMED and is intended to limit additional workload for economic operators already familiar with EU processes. Learn more about the structure of EUDAMED and its specific requirements within this blog.
At present, swissdamed consists of two main modules: the Actors module and the Devices module.
The Actors module went live on August 6, 2024, and it focuses on the registration of economic operators established in Switzerland or Liechtenstein. This includes Swiss or Liechtenstein manufacturers, Swiss authorized representatives acting for foreign manufacturers, and importers based in Switzerland or Liechtenstein. Each operator must register before placing devices on the Swiss market.
Once Swissmedic approves the registration, the operator receives a CHRN (Swiss Single Registration Number). This number serves as a unique identifier and is used across regulatory processes. In practice, the CHRN plays a similar role to the SRN used in the EU, helping authorities clearly identify responsible actors.
The Devices module, also referred to as the UDI module, has been available since August 2025. It is used to register medical devices, in vitro diagnostic medical devices, systems, and procedure packs placed on the Swiss market.
At this stage, device registration is performed via XML file uploads. This approach allows companies to reuse existing data with minimal changes. Looking ahead, Swissmedic plans to introduce machine-to-machine (M2M) interfaces. These will allow automated data exchange and further reduce manual effort for economic operators managing large device portfolios.
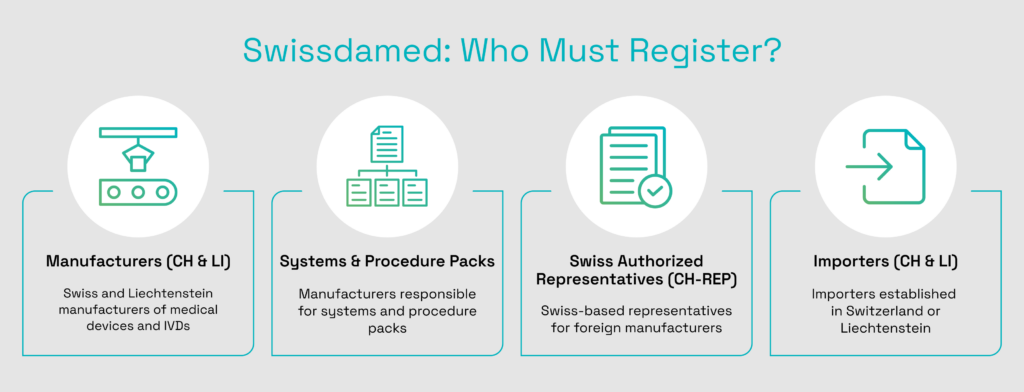
Registration in swissdamed is mandatory for the following economic operators involved in placing medical devices on the Swiss market:
With the osapiens HUB for Medical Devices, you can syndicate validated product data, automate UDI submissions for swissdamed and other global systems (EUDAMED, GUDID, COVIN, and more), and stay deadline-ready with confidence.
swissdamed is closely aligned with the EU’s EUDAMED database, but the two systems are not interchangeable. They are separate regulatory obligations, and companies placing devices on both markets must treat them independently.
EUDAMED serves the EU and EEA and is managed by the European Commission. swissdamed is Switzerland’s national database, also covering Liechtenstein, and is operated by Swissmedic as the competent authority.
EUDAMED is built around six modules, covering actors, devices, certificates and notified bodies, clinical investigations, vigilance, and market surveillance. We dive deeper into these modules in our guide to successful EUDAMED implementation. swissdamed currently focuses on a narrower scope, with the Actors module and the UDI Devices module forming the core of operator and device registration. Further functionality is planned but does not mirror all EU modules.
In EUDAMED, EU manufacturers, authorized representatives, and importers register for the EU market and receive an SRN. In swissdamed, registration is limited to actors established in Switzerland or Liechtenstein.
Both databases use UDI-based and XML-structured data, which makes them conceptually aligned. However, they are technically independent. Data cannot be transferred or synchronized between EUDAMED and swissdamed, meaning companies active in both markets must maintain their data separately. Dive deeper into mastering global UDI compliance, in our hands-on guide.
The swissdamed timeline is drawing close, and the key dates are already set. Companies should plan for July 1, 2026, which is when the device registration becomes mandatory. Registration, data validation, and technical preparation take time, especially as Swissmedic continues to roll out system updates and future M2M interfaces.
To get a clear picture of what the deadlines mean in practice and how to prepare without unnecessary effort, join the osapiens swissdamed webinar on Feb 25, 2026. Within this webinar, medical compliance experts will provide a focused overview of what’s coming, what to prioritize, and how to stay on track before the countdown runs out.
Book your slot now!
Digital Product Passport: Opening of the DPP oLAB in Cologne – Koenig & Bauer and osapiens bridge the Gap between Compliance and Marketing
Sustainability reporting after the Omnibus: What has changed and why reporting still matters
swissdamed Explained: Requirements, Deadlines, and What Medical Device Companies Should Do Now