Community
osapeers.org
EUDAMED will become mandatory on May 28, 2026 — and incomplete or inconsistent product data will directly affect market access, supply chain continuity, and regulatory compliance. The osapiens HUB for Medical Devices provides a pre-validated, fully auditable environment that detects wrong product information to syndicate validated data, automates UDI and product data submissions, and ensures you meet every regulatory requirement before the deadline.
Live in
days
Each regulatory authority has its own database and medical device manufacturers have to satisfy different data formats, data structures, etc.
The data provision via the interface of regulatory authorities is complex, error-prone and lacking any customer experience.
Providing consistent data to all stakeholders (e.g., users, hospitals etc.) is key to maintain trust in the medical device.
In the healthcare sector, managing product information requires navigating complex data formats and protocols for internal use, data exchange, and compliance with stringent quality standards in a validated environment. Enabling trusted healthcare through seamless data exchange, osapiens for Medical Devices facilitates the syndication of medical device data from manufacturers to regulatory databases and trading partners worldwide. Regulations and standards like COVIN, GDSN, EUDAMED, GUDID, LIR, US FDA and others are designed to enhance data quality, ensure measurability, and support electronic processes for quality-assured management.
EUDAMED
Either way, your project will be guided by the most experienced team in the industry. We help you understand, attribute by attribute, what is required and how to meet those regulatory requirements.
If you have done a US FDA GUDID project before, we can help you understand the significant differences in structure and requirements between the two, including understanding and meeting EUDAMED data structures with BUDI.
Contact us today for a demo or to discuss the best project plan for your organization.


Get a clear and practical overview of the UDI requirements and processes. Learn how to meet regulatory demands efficiently and benefit from digital support.
Get a clear and practical overview of the EUDAMED requirements and processes. Learn how to meet regulatory demands efficiently and benefit from digital support.
osapiens for Medical Devices streamlines the transfer of medical device data, ensuring seamless exchange between manufacturers, regulatory databases, and trading partners worldwide. The BYRD data integration solution helps you manage product information effortlessly, while the BYRD connector solution ensures compliance by automatically aligning your data with the required formats of global regulatory authorities. Eliminate friction in your data exchange and gain the trust of your partners and customers.
Ensure your data reaches regulatory authorities and business partners in the correct format — without manual effort.
Our validated environment and stringent security measures ensure that you have a secure and compliant process that meets QMS requirements for registering and exchanging your UDI data with your trading partners.
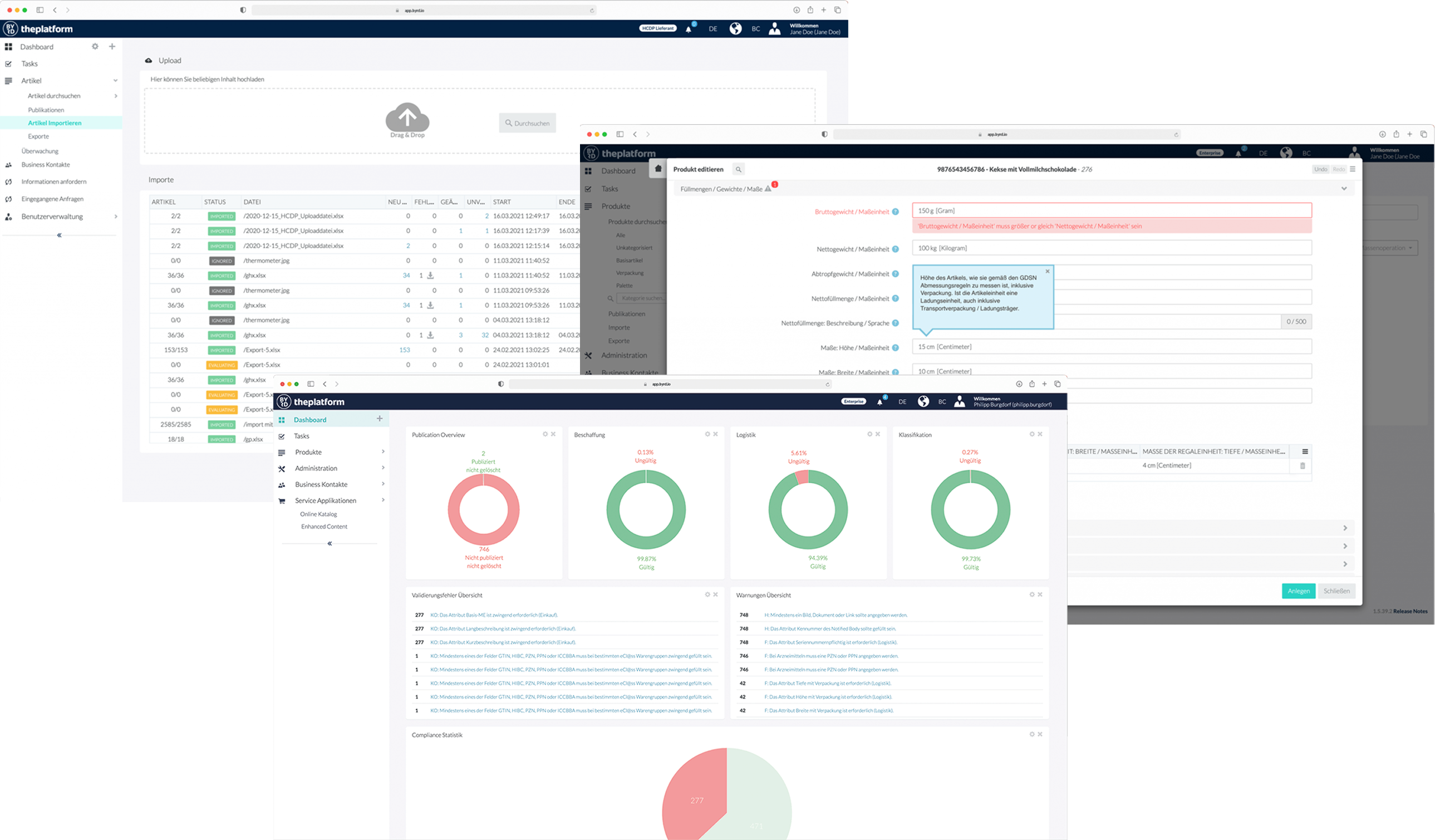
The osapiens platform supports multiple methods of data transfer, including Machine to Machine (XML via AS/2, API , JSON), Excel uploads, and manual entry. The osapiens platform allows you to manage your data and Syndication in 1 place connecting you to GS1/GDSN, EUDAMED, FDA GUDID, GUID, TGA Australia, Swissmedic, Emirates Health Services (EHS), Dubai Health Authority (DHA) and others.
We are committed to providing connectivity from osapiens to all Federal Entities with UDI databases. In the future, when the Regulatory bodies establish their programs we expect to provide connectivity to Colombia/INVIMA, Brasil/ANVISA, UK/NHS & MHRA, Canada, Singapore, and Brazil.
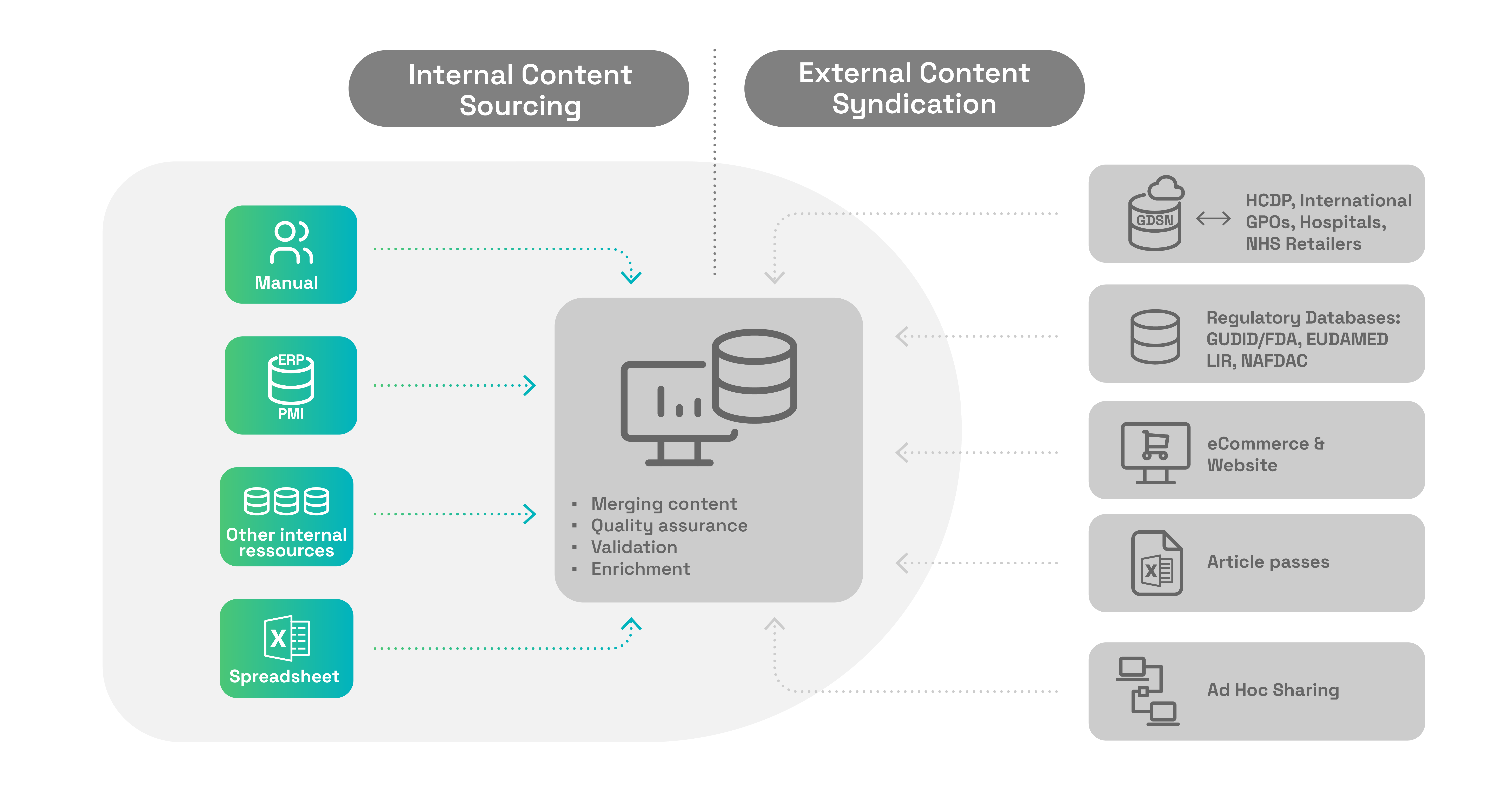
osapiens for Medical Devices supports the entire Product Content Life Cycle Management
Aggregation
Content and product data aggregation within the osapiens for Medical Devices solution allows you to centrally manage all required product information and keep it globally compliant. By aggregating your data, you ensure that your products meet all regulatory requirements - efficiently and error-free.
Benefits of aggregating product data:
Take advantage of centralized data aggregation and ensure efficient and compliant management of your medical devices. Stay UDI-compliant and maximize your efficiency!




Nowhere else is quality-assured product information as important as in the healthcare sector. For manufacturers, purchasing groups, and other market participants, there are stringent requirements that can only be met with the right technology and optimised processes.





